In the frame of the Joint Transnational call 2020, Bruno Amati (Department of Experimental Oncology) and Enrico Derenzini (Division of Clinical Hemato-Oncology) win an Era Permed grant to explore innovative personalised cancer therapy approaches to treat high risk lymphoma patients.
Era PerMed fosters excellence and interdisciplinary and international collaboration, and encourages translational research.
In this framework, an interdisciplinary and international consortium -which includes Italian, German and French partners- coordinated by Bruno Amati and Enrico Derenzini at IEO, aims at defining a new personalized cancer therapy approach targeting minimal residual disease, namely the few cells that survive therapy and are responsible for cancer relapse, in Diffuse Large B-cell lymphoma (DLBCL).
DLBCL is a cancer arising from B-lymphocytes (a subtype of white blood cells), which is currently treated by immuno-chemotherapy. However, current approaches only cure about 60% of the patients, while others develop resistance to therapy and eventually relapse. Therapy resistance results from cancer-associated mutations that may be very different from patient to patient: targeting those mutations is the goal of personalized medicine.
The contemporary presence of translocations activating the MYC and BCL2 oncogenes results in the so-called “double-hit” lymphoma (DHL), a particularly aggressive and high-risk lymphoma subtype.
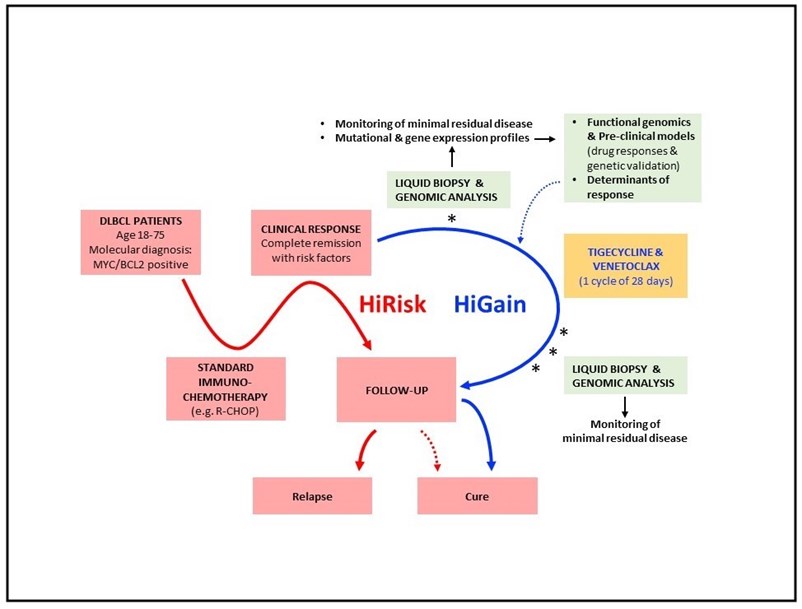
Amati’s lab has previously shown that combined treatment with two drugs, Venetoclax and Tigecycline, is able to completely eradicate the disease in animal models of double-hit lymphoma. Following up on these results, the aim of this grant is to translate those findings into the clinics. The main goal will be to assess safety and effectiveness of Venetoclax/Tigecycline treatment following standard therapy, with the aim to eradicate minimal residual disease, thus preventing relapse. Liquid biopsies (e.g. patient blood samples) will be collected after standard therapy and Venetoclax/Tigecycline treatment, and will be molecularly analyzed to assess the presence of residual cancer cells. Moreover, mutational profiling of the same samples will enable to identify the distinctive features (e.g. biomarkers) of the responsive vs. non-responsive tumors. These findings will be a step forward towards the introduction of Venetoclax/Tigecycline as a personalized therapeutic approach in routine healthcare.
Figure legend. The HiRisk-HiGain trial will address whether complementation of standard immuno-chemotherapy (red line) by a consolidation treatment with Tigecycline and Venetoclax increases the frequency of disease eradication – i.e. cure – in MYC/BCL2-associated diffuse large-B cell lymphoma (DLBCL). Patients will be enrolled based on positivity for MYC and BCL2 (with either IHC or FISH) and the achievement of primary remission with remaining risk factors (slow responders or detectable minimal residual disease), and will include a subset of «double-hit» cases with dual translocations. Repeated liquid biopsy (blood sampling) and monitoring of minimal residual disease shall allow to anticipate eventual relapse and to unravel the genetic correlates (or biomarkers) of treatment response. Complementary experiments in pre-clinical models (mice) shall validate those biomarkers and highlight the mechanisms connecting them to treatment response.