NUMB protein is a tumor suppressor and its loss of function is involved in cancer development in many tumor types. Its role has been analyzed in depth in breast, where it has been shown to be critically involved in stem cell physiology. NUMB loss –which can occur through a number of different mechanisms–, by altering stem cell division (from asymmetric to symmetric), leads to deregulated expansion, typical of cancer stem cells.
In a recent paper by Sabbioni, Filippone et al, the authors, headed by Daniela Tosoni –researcher at the department of experimental oncology of IEO and at the university of Milan–, Pier Paolo Di Fiore and Salvatore Pece, revealed a novel vulnerability of breast cancer cells, demonstrating that the CRL7FBXW8 protein complex controls stem cell proliferation through NUMB regulation: specifically, CRL7FBXW8 is involved in the aberrant NUMB degradation in breast cancer. CRL7FBXW8 inhibition is sufficient to restore the normal NUMB levels and thus counter tumor growth, proposing CRL7FBXW8 as a potential new therapeutic target in breast cancer.
The same research group has previously demonstrated, in preclinical studies, that interfering with NUMB levels can arrest tumor growth. However, when these approaches have been tested in clinical trials, researchers faced a limited efficacy and high toxicity. The results of this study propose a different, likely more precise, strategy to interfere with NUMB activity which, by acting on an upstream factor regulating NUMB levels –the CRL7FBXW8 protein complex– may allow to achieve greater efficacy with lower toxicity. The drug Pevonedistet may be exploited to interfere with CRL7FBXW8 activity. Pevonedistat is being currently tested in clinical trials for the treatment of hematologic and solid tumors. Pevonedistat treatment of some breast cancer subtypes, selected on the basis of NUMB presence/absence as biomarker, is an approach that certainly deserves to be further explored.
“Although NUMB restoration strategies have not yet been implemented in clinical practice –comments Daniela Tosoni–, this study provides strong preclinical evidence that proteasome inhibition, such as with bortezomib, may effectively stabilize NUMB levels and impair cancer stem cell expansion. While bortezomib is currently approved for hematological malignancies, its potential repurposing in biomarker-defined subsets of breast cancer warrants further investigation.”
“We propose a refined therapeutic strategy targeting upstream regulators of NUMB degradation –adds Maria Grazia Filippone– which may offer enhanced specificity and improved therapeutic windows. In particular, Pevonedistat (MLN4924), a neddylation inhibitor currently under clinical evaluation for hematologic and solid tumors, may effectively inhibit CRL7FBXW8 activity. Its use in NUMB-deficient breast cancer, guided by Numb loss/biomarker-based patient stratification, represents a compelling avenue for future clinical development.”
While physiologically controlling stem cell proliferation, NUMB loss of function is associated with tumorigenesis. Indeed, the authors found that while being highly abundant in healthy mammary tissue, its expression in patients’ breast cancer tissues was highly variable and, in some cases, completely lacking. NUMB deficiency in tumor tissue correlated with increased mortality. The remarkable difference in NUMB protein levels in healthy as compared to tumoral tissues parallel with an only slight difference in terms of RNA levels indicated a post-transcriptional/post-translational regulation, in line with previous preliminary data suggesting a ubiquitin degradation-mediated modulation of NUMB levels.
The mechanism. Analyses of the molecular mechanisms underlying NUMB degradation suggested the involvement of the CRL7FBXW8 protein complex. Indeed, firstly, in NUMB-deficient cells, NUMB expression could be restored by either inhibition of proteasome-mediated degradation by bortezomib, or by MLN-mediated inhibition of CRL complexes; secondly, siRNA-based screening revealed the critical involvement of two components (RBX1 and FBXW8) of the CRL7FBXW8 complex in ubiquitin-mediated degradation (specifically, in ubiquitin ligation to the target protein to be degraded). Indeed, silencing of these two components of the CRL7FBXW8 protein complex resulted in the reduced ubiquitin-mediated NUMB degradation; the resultant higher NUMB levels had a functional impact on NUMB-related cell pathways. Mechanistically, CRL7FBXW8 protein complex physically interacted with NUMB, in turn inducing its hyper-degradation.
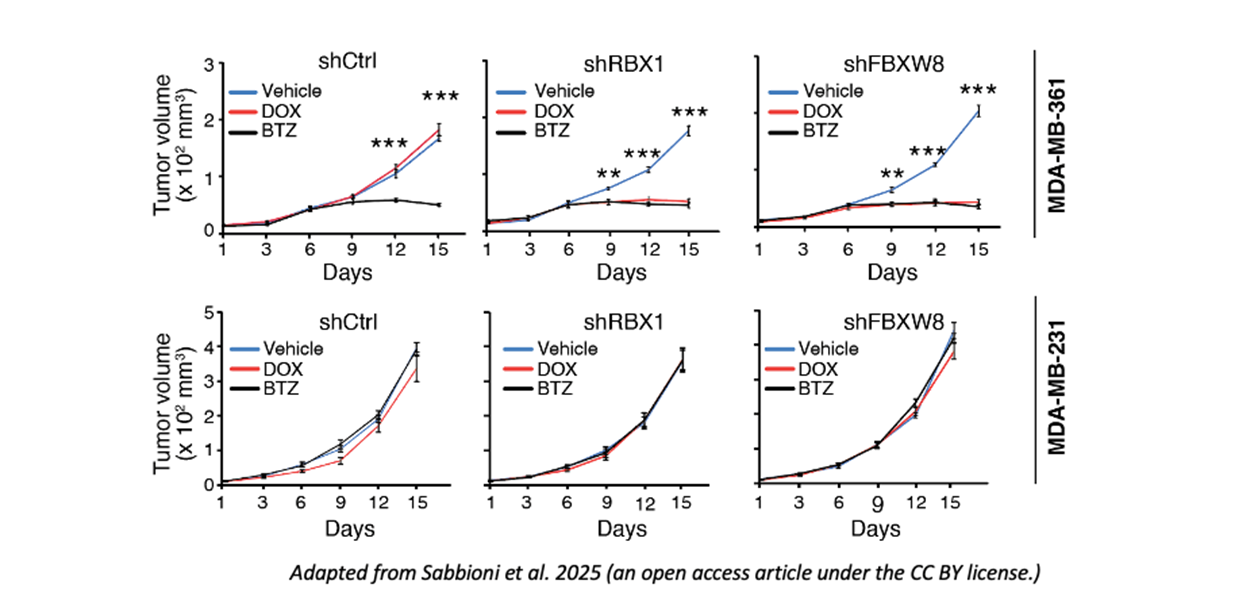
Functional impact of NUMB hyper-degradation. Once defined the (CRL7FBXW8-related) molecular mechanism regulating NUMB degradation –and abundance–, they explored the biological consequences. They found that pharmacological inhibition (by bortezomib of MLN) or silencing of the two components of the CRL7FBXW8 complex, by preventing NUMB degradation and thus resulting in higher NUMB levels, damaged stem cell self-renewal, thus impairing tumor growth, in vitro and in vivo, indicating that bortezomib and MLN can selectively reduce the tumorigenic potential and number of NUMB-deficient cancer stem cells. They also demonstrated (through the silencing of CRL7FBXW8 complex components and the measurement of tumor growth) that NUMB-deficient cells were highly tumorigenic due to the hyper-activity of CRL7FBXW8 and the ensuing NUMB hyper-degradation.
Clinical implications. Finally, they explored the clinical relevance of the identified correlation between CRL7FBXW8-mediated NUMB degradation and tumorigenicity. They established human in vivo preclinical models (PDX) by transplanting patient-derived NUMB-proficient and NUMB-deficient tumor cells in murine recipients. NUMB-deficient tumors were sensitive to either bortezomib treatment of CRL7FBXW8 silencing, which reduced NUMB degradation and restored NUMB levels, resulting in reduced tumor growth.
In the cancer stem cell compartment specifically, the loss of both components of the CRL7FBXW8 complex, resulting in the loss of NUMB degradation, reduced cancer stem cell number.
By demonstrating that CRL7FBXW8 complex-mediated hyper-degradation of NUMB induces tumor onset, these results propose a novel potential therapeutic target in breast cancer.