Overweight and type2 diabetes (T2D) women surviving breast cancer are at higher risk of recurrence. Insulin and estrogen levels as well as inflammation have been linked to tumor growth. Insulin involvement in tumor growth has led to hypothesize that interfering with insulin signaling, with metformin (a common anti-diabetic drug), could counter tumor progression. Indeed, antitumor effects of metformin have been observed in obese and T2D patients.
Is metformin also effective in breast cancer relapse?
By using patient data collected within the MetBreCS trial –in which researchers performed a transcriptional analysis of breast tissue from pre- and post- menopausal breast cancer survivors receiving metformin– the authors, including IEO researchers Sara Gandini, Bernardo Bonanni, Harriet Johansson and Federica Bellerba, evaluated whether metformin reduced cell proliferation of healthy breast tissue of obese survivors. Through the analysis of gene expression profile of patients at baseline and at the end 1-year metformin administration, and the integration of such data with information regarding blood levels of steroid hormone and metabolites, their final goal was the identification of pathways altered by metformin treatment.
With this work, the authors investigated, for the first time, by gene expression analysis, the potential preventive effect of metformin administration in breast cancer relapse. Their results suggest that metformin might affect the expression levels of some genes in breast tissue that prevent cancer initiation, thus avoiding cancer recurrence, as well as modulate the systemic levels of some metabolites in turn involved in cancer growth.
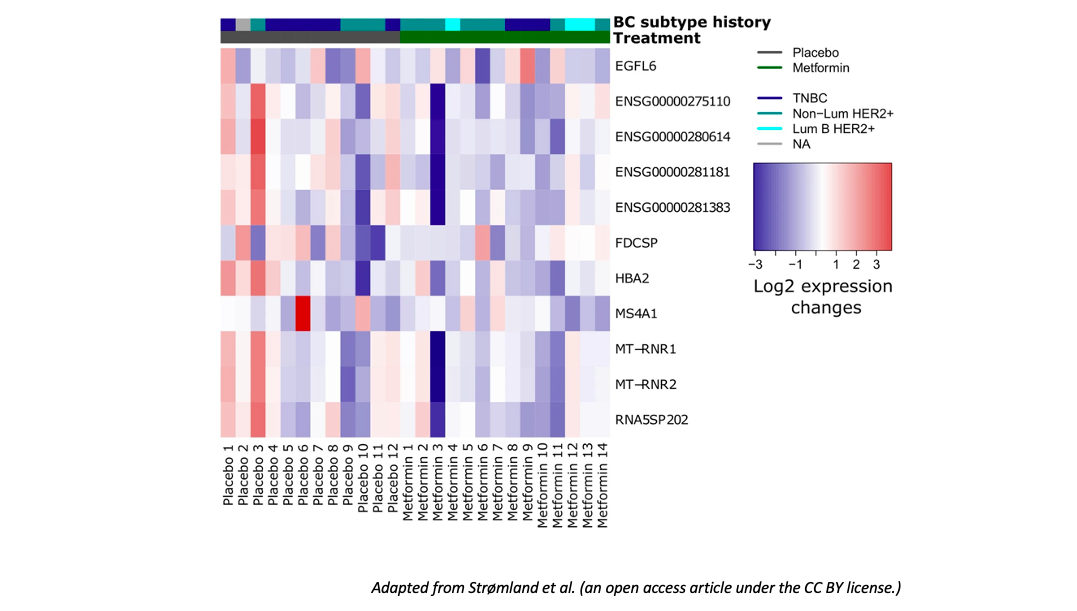
To define the effect of metformin on breast tissue gene expression in post-menopausal women, the authors performed RNAseq before and after metformin administration. Gene expression analyses at the end of 1-year metformin treatment showed a different expression level –as compared to placebo-treated patients– of some genes: MS4A1, MT-RNR1, MT-RNR2, HBA2 were downregulated after metformin administration, while EGFL6 and FDCSP were downregulated in the placebo group. EGGL6 gene is associated with cancer cell proliferation and migration and promotes epithelial-to-mesenchymal transition. FDCSP gene is typically overexpressed in ovarian, endometrial, breast, prostate tumors, where it contributes to cell migration and metastatic dissemination.
Then, they measured metabolite levels in the blood. They found, after metformin treatment, a decrease of arginine and citrulline. Arginine is synthesized from citrulline. Although high arginine levels are known to be correlated with greater antitumor immune system activity, arginine is also known to modulate several tumor-related processes. Indeed, their analyses revealed a reduced cell proliferation associated with the lower citrulline levels following metformin treatment.
Estrogen levels in the blood were also decreased after metformin administration, as well as several other metabolites, whose levels specifically correlated with the lower estrogen levels. The blood metabolites whose altered levels after metformin were specifically linked with estrogens, in turn correlated with the (metformin-related increased) expression of genes such as CYP11A1 and CYP1B1, which are known to regulate the metabolism of steroid hormones.